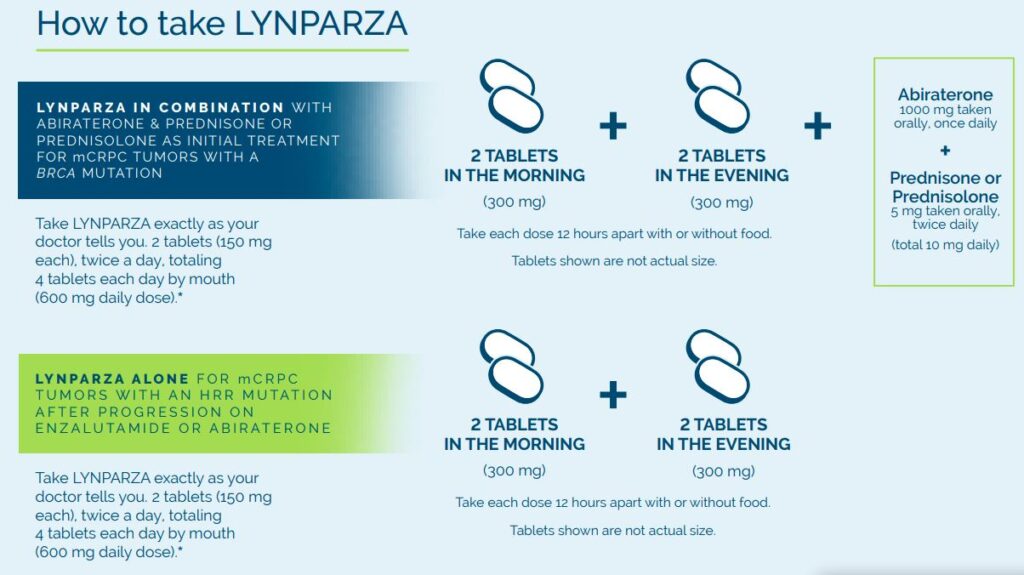
LYNPARZA in combination with abiraterone and prednisone or prednisolone may be for you:
- If your prostate cancer is metastatic, which means it has spread to other parts of your body
- If your prostate cancer no longer responds to treatment that lowers testosterone (castration resistant)
- If you have a certain type of abnormal inherited or acquired BRCA mutation
Your healthcare provider will perform a test to make sure that LYNPARZA is right for you.
LYNPARZA alone may be for you:
- If your prostate cancer has certain HRR (homologous recombination repair) gene mutations
- If your prostate cancer is metastatic, which means it has spread to other parts of your body, and no longer responds to treatment that lowers testosterone (castration resistant)
- If your cancer has progressed after prior treatment with certain hormone therapies, enzalutamide or abiraterone
Your healthcare provider will perform a test to make sure that LYNPARZA is right for you.
Top 5 Most Common Side Effects:
- Nausea: Occurs in approximately 60% to 77% of patients.
- Fatigue (Tiredness) or Weakness: Reported by about 55% to 67% of patients.
- Anemia (Low Red Blood Cell Counts): Experienced by approximately 36% to 46% of patients.
- Vomiting: Occurs in about 32% to 40% of patients.
- Diarrhea: Reported by approximately 24% to 34% of patients.
Top 5 Most Serious Side Effects:
- Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML): These are rare but serious bone marrow disorders that have been reported in a small number of patients. The exact incidence is not well-defined but is considered uncommon.
- Pneumonitis (Lung Inflammation): This is an uncommon but serious side effect. The exact incidence rate is not specified but is considered rare.
- Blood Clots (Venous Thromboembolism): Some patients may develop blood clots, including deep vein thrombosis or pulmonary embolism. The exact incidence is not specified but is considered uncommon.
- Severe Bone Marrow Suppression: This can lead to significantly low blood cell counts, increasing the risk of infections, anemia, or bleeding. The exact incidence is not specified but is considered uncommon.
- Severe Allergic Reactions: While rare, severe allergic reactions can occur. The exact incidence is not specified but is considered rare.
Factors to Consider:
- Genetic Testing: Lynparza is most effective in patients with specific BRCA1/BRCA2 mutations or other homologous recombination repair (HRR) gene mutations. Genetic testing is crucial to determine if you carry these mutations.
- Cancer Type and Stage: Lynparza is typically used in advanced or metastatic cancers that have progressed after other treatments.
- Previous Treatments: Your eligibility may depend on the therapies you’ve already tried, such as chemotherapy or hormone therapy.
- Side Effects Tolerance: Lynparza can have significant side effects, including nausea, fatigue, anemia, and, rarely, severe complications like myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).
- Willingness to Monitor: Regular blood tests and follow-ups are required while taking Lynparza to monitor your blood counts and ensure no serious side effects develop.
Who Shouldn’t Use Lynparza:
- Individuals without relevant genetic mutations (e.g., BRCA1/BRCA2 or HRR mutations) may not benefit significantly from Lynparza.
- Patients with certain health conditions (e.g., severe liver or kidney impairment) may need to avoid Lynparza or use it with caution.
- Pregnant or breastfeeding individuals should not use Lynparza due to potential harm to the fetus or infant.